Which
of the following is true for virtual equation of state
(A) Virial coefficients are universal
constants
(B) Virial coefficient B represents three
body interactions
(C) Virial coefficients are functions of
temperature only
(D) For some gases, virial equations and
ideal gas equations are the same
GATE
1999
Answer: (C)
Max Well’s relation corresponding to the identity dH = TdS + VdP + ∑μidni is
GATE
1999
Answer: (D)
A
gas mixture of three components is brought in contact with a dispersion of an
organic phase in water. The degrees of freedom of system are
(A) 4
(B) 3
(C) 5
(D) 6
GATE
1999
Answer: (C)
On
a P-V diagram of an ideal gas, suppose a reversible adiabatic line intersects a
reversible isothermal line at point A. Then at point A, the slope of the
reversible adiabatic line (∂P/∂V)T are related as
GATE
2000
Answer: (C)
The
thermal efficiency of a reversible heat engine operating between two given
thermal reservoirs is 0.4. The device is used either as a refrigerator or as a
heat pump between the same reservoirs. Then the coefficient of performance as a
refrigerator (COP)R and the coefficient of performance of as a heat
pump (COP)HP are
(A) (COP)R = (COP)HP
=0.6
(B) (COP)R = 2.5
(C) (COP)R = 1.5; (COP)HP
=2.5
(D) (COP)R = (COP)HP
=2.5
GATE
2000
Answer: (C)
The
max Well relation derived from the differential expression for the Helmoltz
free energy (dA) is
GATE
2001
Answer: (D)
At
100⁰C, water and methylcyclohexane both have
vapour pressures of 1.0 atm. Also at 100⁰C,
the latent heats of vapourisation of these compounds are 40.63 KJ/mole for
water and 31.55 KJ/mole for methyl cyclohexane. The vapour pressure of water at
150⁰C 4.69 atm. At 150⁰C,
the vapour pressure of methyl cyclohexane would be expected to be
(A) Significantly less than 4.69 atm
(B) Nearly equal to 4.69 atm
(C) Significantly more than 4.69 atm
(D) Indeterminate due to lack of data
GATE
2001
Answer: (A)
Air
enters an adiabatic compressor at 300 K. The exit temperature for a compressed
ratio of 3, assuming air to be an ideal gas. (γ = CP/CV =
7/5) and the process to reversible is
(A) 300(32/7)
(B) 300(33/5)
(C) 300(33/7)
(D) 300(35/7)
GATE
2001
Answer: (A)
Which
of the following conditions are satisfied at the critical point by the P-V-T
relation of a real fluid
GATE
2002
Answer: (A)
The
number of degrees of freedom for an azeotropic mixture of ethanol and water in vapour-liquid
equilibrium, is
(A) 3
(B) 1
(C) 2
(D) 0
GATE
2002
Answer: (B)
The
partial molar enthalpy of a component in an ideal binary gas mixture of
composition z, at a temperature T and pressure P. is a function only of
(A) T
(B) T and P
(C) T, P and z
(D) T and z
GATE
2002
Answer: (A)
Which
of the following identities can be most easily used to verify steam table data
for superheated steam
(A)
(∂T/∂V)V = -(∂P/∂S)V
(B)
(∂T/∂P)S = (∂V/∂S)P
(C)
(∂P/∂T)V = (∂S/∂V)T
(D)
(∂V/∂T)P = -(∂S/∂P)T
GATE
2002
Answer: (D)
Steam
undergoes isentropic expansion in a turbine from 5000 kPa and 400°C (entropy =
6.65 kJ/kg K) to 150 kPa (entropy of saturated liquid = 1.4336 kJ/kg K, entropy
of saturated vapour = 7.2234 kJ/kg K). The exit condition of steam is
(A) superheated vapour
(B) partially condensed vapour with quality
of 0.9
(C) saturated vapour
(D) partially condensed vapour with quality
of 0.1
GATE
2002
Answer: (B)
1 m3
of an ideal gas at 500 K and 1000 kPa expands reversibly to 5 times its initial
volume in an insulated container. If the specific heat capacity (at constant
pressure) of the gas is 21 J/mol K, the final temperature will be
A.
35 K
B.
174 K
C.
274 K
D.
154 K
GATE
2002
Answer: (B)
One
mole of Nitrogen at 8 bar and 600 K is contained in a piston-cylinder
arrangement. It is brought to 1 bar isothermally against a resisting pressure
of 1 bar. The work done (in Joules) by the gas is
(A) 30554
(B) 10373
(C) 4988.4
(D) 4364.9
GATE
2003
Answer: (B)
A
steam turbine operates with a superheated steam flowing at 1 kg s-1.
This steam is supplied at 41 bar and 500°C, and discharges at 1.01325 bar and
100°C
Data:
41 bar, 500°C
Enthalpy:
3443.9 kJ kg-1
Entropy:
7.0785 kJ kg-1K-1
41
bar, 251.8 °C
Enthalpy
of saturated steam: 2799.9kJ kg-1
Entropy
of saturated steam: 6.0583kJkg-1K-1
1.01325
bar 100°C
Enthalpy
of saturated vapour: 2676 kJ kg-1
Enthalpy
of saturated liquid: 419.1 kJ kg-1
Entropy
of saturated vapour: 7.3554 kJ kg-1 K-1
Entropy
of saturated liquid: 1.3069 kJ kg-1 K-1
The
maximum power output (in kW) will be
(A) 644.0
(B) 767.9
(C) 871.3
(D) 3024.8
GATE
2003
Answer: (C)
In
Joule’s experiments, an insulated container contains 20 kg of water initially at
25°C. It is stirred by an agitator, which is made to turn by a slowly falling
body weighing 40 kg through a height of 4 m. The process is repeated 500 times.
The acceleration due to gravity is 9.8 ms-2 Neglecting the heat
capacity of agitator, the temperature of water (in °C) is
(A) 40.5
(B) 34.4
(C) 26.8
(D) 25
GATE
2003
Answer: (B)
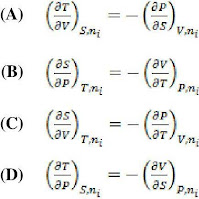



No comments:
Post a Comment